Mouse Embryo Culture Chamber and Imaging System and Methods of Use
The culture of mouse embryos ex utero and continuous monitoring and imaging of embryos as they develop have applications in drug testing, genetic studies, and basic research on embryonic development. However, the embryo culture systems currently available for post-implantation embryos include rolling bottle culture systems, which do not permit imaging of the developing embryos and do not support the long-term survival and development of embryos ex utero. Current culture systems for pre-implantation stage embryos, including microfluidic culture systems, are suitable for short-term culture of very early stage embryos – such as fertilized zygotes. However, they are not suitable for the culture and survival of post-implantation stage embryos. Similarly, the cell culture systems currently available (e.g., slides, multi-well plates, and microfluidic chambers) may allow for imaging of single-dimensional structures such as cells, but cannot be used for culturing or imaging three-dimensional structures such as embryos. Therefore, there is a need for a system that provides long-term embryo culturing combined with imaging. Such a system could support long-term culture while allowing for continuous imaging of the developing embryos.
Scientists at the National Eye Institute (NEI) have developed an embryo culture chamber, an embryo culturing and imaging system, and a method of culturing and imaging an embryo. The chamber allows for the continuous imaging of the embryo for the culture period. This invention is available for licensing and co-development.
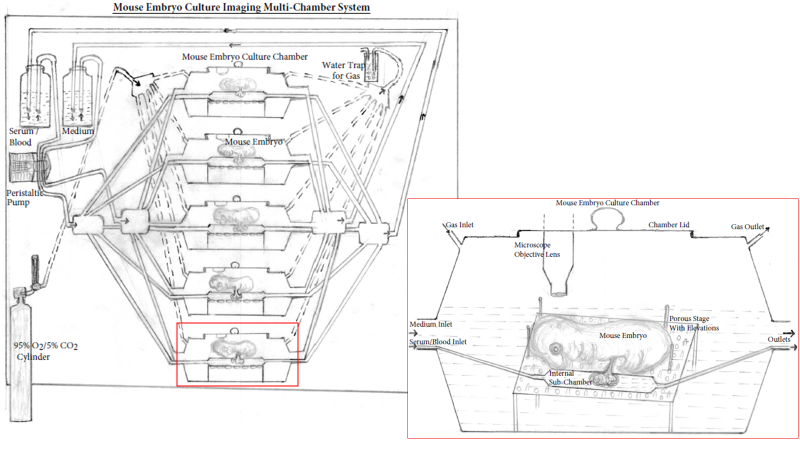
Figure: Mouse embryo culture imaging multi-chamber system. The multi-chamber mouse embryo culture imaging system consists of multiple embryo culture chambers connected to a common medium and gas (95% O2/5% CO2) source. Culture medium (and serum/blood) flows through the peristaltic pump into the culture chamber (blood/serum flows through internal sub-chamber) submerging the embryos and passes through the outlets to recirculate. Gas flow through the chambers can be monitored as it passes through the water trap and adjusted by a flow meter. The inlet shows enlarged view of the culture chamber with a microscope objective lens for imaging and a removable chamber lid. Mouse embryos are cultured on specialized platform with pores and elevations to provide minimal contact with the surface and allow free flow of medium in all directions. The serum/blood flowing through the internal sub-chamber baths the mouse placenta/yolk sac simulating placental blood supply.
Competitive Advantages:
- The internal sub-chamber simulates placental blood supply through which blood/serum can be supplied to developing embryos
- The unique design of the stage/platform on which the embryo rests enables maximum exposure of the body surface area to the circulating medium and oxygen permits appropriate development and survival of the embryos long-term
- The embryo culture chamber enables continuous imaging of developing embryos during culture
- Development of specific organ systems, such as eye, lungs, heart, liver, etc., can be monitored and imaged
- Temporal and spatial distribution of pharmacological molecules (e.g., molecules/proteins tagged with fluorescent markers) in different organ systems and their effect on a specific organ or whole-body development can be monitored and studied
- The blood/serum supply through the internal sub-chamber simulates placental blood supply and can be used to treat embryos with specific drugs/molecules while the litter mates serve as controls for studying the effect of specific molecules on organ system development
- Long-term or complete ex utero development of post-implantation stage embryos provides access to developing mammalian embryos for pharmacological manipulation and research studies
Commercial Applications:
- Drug screening for teratogenic and developmental effects with temporal and spatial evaluation on different organ systems during embryonic growth
- Substituting cell-culture and organoid based culture systems as direct testing or studies on mammalian embryos (e.g., mouse embryos)
- Genetic screening for developmental defects with direct visualization of the embryonic development
- Drug development and evaluation of molecules regardless of whether they cross the placental barrier
- Visualization of the developing organ systems and tissues at the embryonic level
