Opportunity for Infectious Disease Diagnostic Test That Improves Safety and Lowers Cost

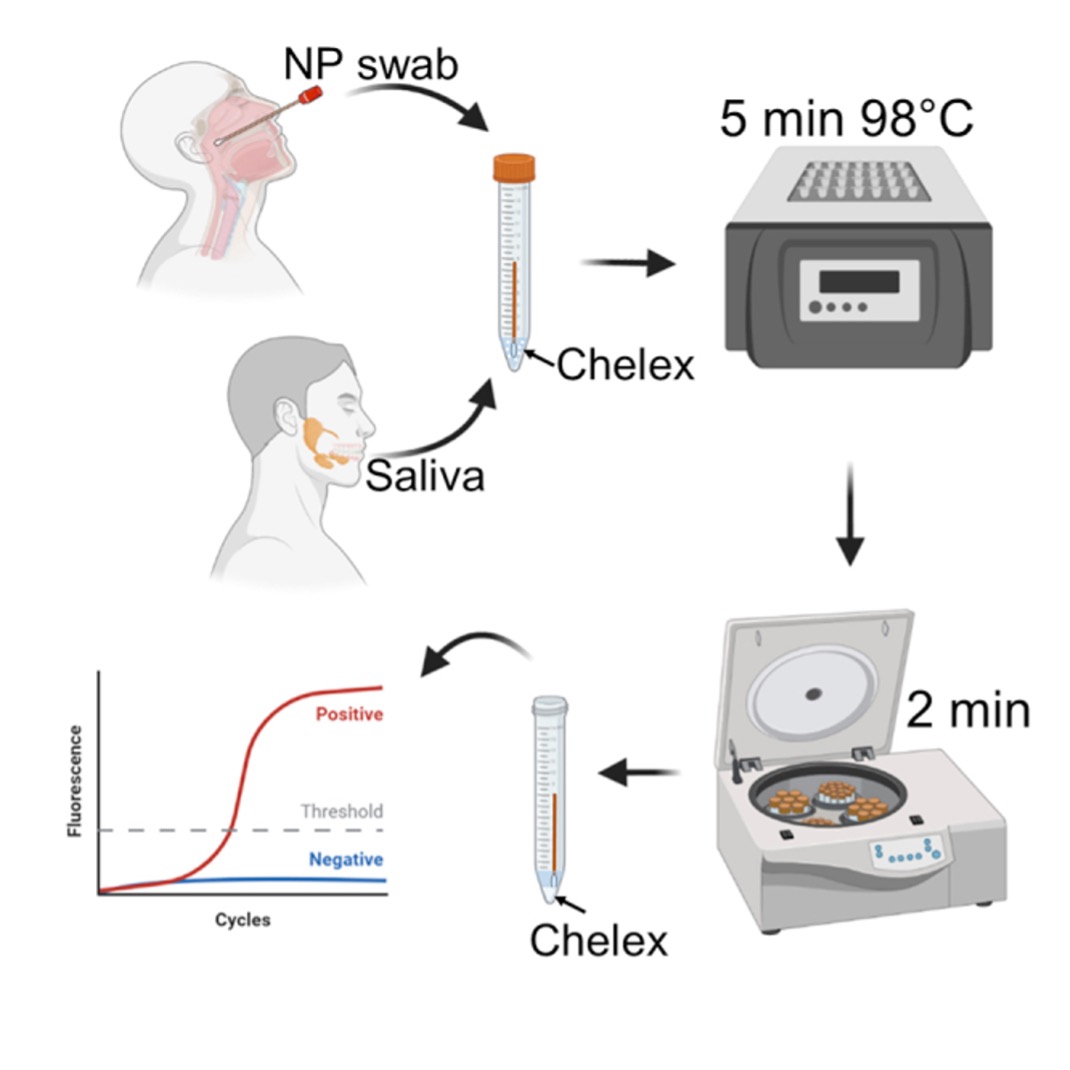
Current diagnostic tests for COVID-19 and other viruses require RNA extraction and analysis of the extracted RNA sample. NIH Inventors have developed a novel, improved sample preparation method that eliminates the need for an RNA extraction step. The inventors discovered that incorporating a chelating agent into the RT-qPCR heating step ties up the magnesium and calcium ions needed for RNase activity, thereby increasing the amount of RNA produced for analysis. It also removes potential inhibitors of RT-qPCR and inactivates SARS-CoV-2 infectivity, which improves workflow safety and eliminates the need for a BSL-2 testing facility.
This is a versatile and safe method for RNA preparation for a variety of patient samples and beyond the SARS-CoV-2 virus. It is suitable for standard clinical collection and testing on high throughput platforms for both DNA and RNA.
Competitive advantages of this technology include:
- Improved workflow safety,
- Removes potential inhibitors of RT-qPCR,
- Inactivates SARS-CoV-2 infectivity, and
- Eliminates the need for an RNA extraction step.
Potential commercial applications of this technology include:
- Improved COVID-19 diagnostic test,
- Improved DNA or RNA-based diagnostic test for additional infectious diseases,
- Safer preparation of patient samples, and
- Reagent kits for biomarker profiles and inherited diseases.
Inventors from the NIH National Eye Institute (NEI) are seeking research and co-development partners and/or licensees to:
- Advance the production and uses of the new RNA preparation method,
- Manufacture reagent kits for testing in patients with suspected COVID-19 and other DNA/RNA viruses, and
- Manufacture reagent kits for patient biomarker profiles and inherited disease diagnostics.
If you are interested in learning more about this technology or speaking with the licensing manager, please view the abstract: Sensitive and Economic RNA Virus Detection Using a Novel RNA Preparation Method.
