Featured Research - Two Opportunities from NHGRI
NHGRI Seeking Partner for Phase II Trial for Kidney and Diabetes Therapeutic
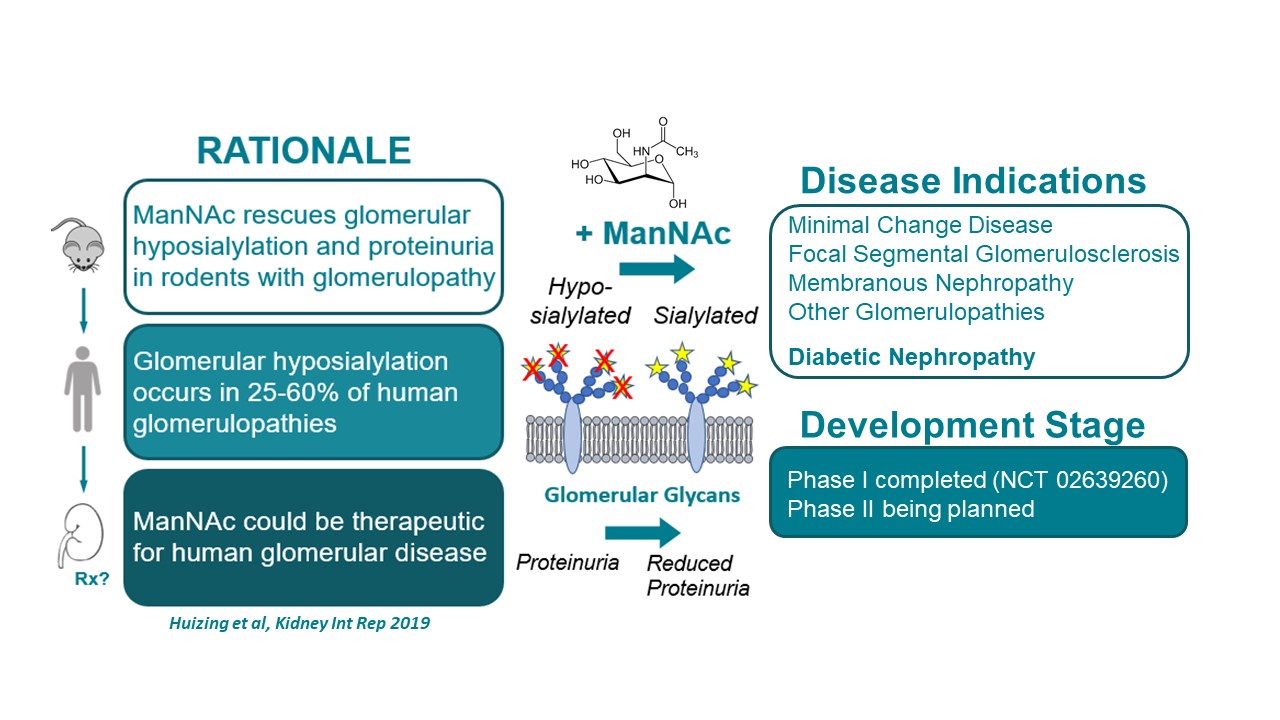
Inventors at the National Human Genome Research Institute (NHGRI) discovered that N-acetylmannosamine (ManNAc) can be used for therapeutic purposes. ManNAc therapy given orally shows long-term safety and biochemical efficacy.
ManNAc is a small uncharged physiological molecule that crosses membranes readily. It is an intracellular precursor of sialic acid synthesis. NHGRI discovered that it can be used to treat certain kidney diseases (such as those involving proteinuria and hematuria) that are a result of a lack of sialic acid.
This therapeutic could be used to treat kidney disorders due to sialic acid deficiency, including but not limited to minimal change disease glomerulopathy, focal segmental glomerulosclerosis, and membranous nephropathy in humans. ManNAc therapy might also be used to treat diabetic nephropathy or diabetes.
NHGRI is seeking a partner for a Phase II clinical trial of ManNAc therapy. Previous clinical research and the Phase I clinical trial found that:
- ManNAc is easy to administer to patients.
- Long-term administration has been shown to be safe and well-tolerated in humans.
Other advantages of this technology include that there are many issued patents in the U.S., Canada, Europe, Japan, and Israel for this indication. There are also extensive published and unpublished preclinical data for the kidney indication available.
NHGRI is looking for both a licensee and a clinical Cooperative Research and Developments Agreement (CRADA) collaborator for this therapeutic. The Phase II study is currently being developed and will be held at NIH. NHGRI will provide their expertise, recruit patients for the study, and is open to transferring their Investigational New Drug (IND) filing.
If you are interested in partnering with NHGRI for a Phase II Clinical Trial of ManNAc therapy, please see this abstract for further information and the licensing contact: Treating Kidney Disorders and Diabetic Nephropathy with N-acetyl mannosamine (ManNAc).
New Vitamin B12 Injectables Available for Licensing or Collaboration
Cobalamin C deficiency (cblC), caused by mutations in the MMACHC gene, is the most common inborn error of intracellular vitamin B12 metabolism. Inventors at the National Human Genome Research Institute (NHGRI) have developed a novel combination of hydroxo- and methylcobalamin, which has shown superior performance compared to traditional hydroxocobalamin only treatment. This technology enables the use of new doses and formulations of cobalamin (vitamin B12) for diseases with limited treatment options. NHGRI has generated MMACHC knockout mouse models. The clbC mice have enabled proof of concept testing with traditional hydroxocobalamin formulations and doses.
NHGRI is searching for a licensee or collaboration partner to use these results and models to formulate and test new cobalamin preparations (injectables) for the treatment of a large group of inborn errors of metabolism, neurological, ocular, and vascular disorders. This technology could also be used to treat primary or secondary vitamin B12 deficiency, nutritional conditions, hyperhomocysteinemia, thrombotic microangiopathies, and possibly behavioral conditions.
Further information and a licensing contact can be found on the abstract: High Concentration Methylcobalamin or Combination of Methyl- and Hydroxocobalamin for the Treatment of Cobalamin C Deficiency and Related Disorders.
